Product Information
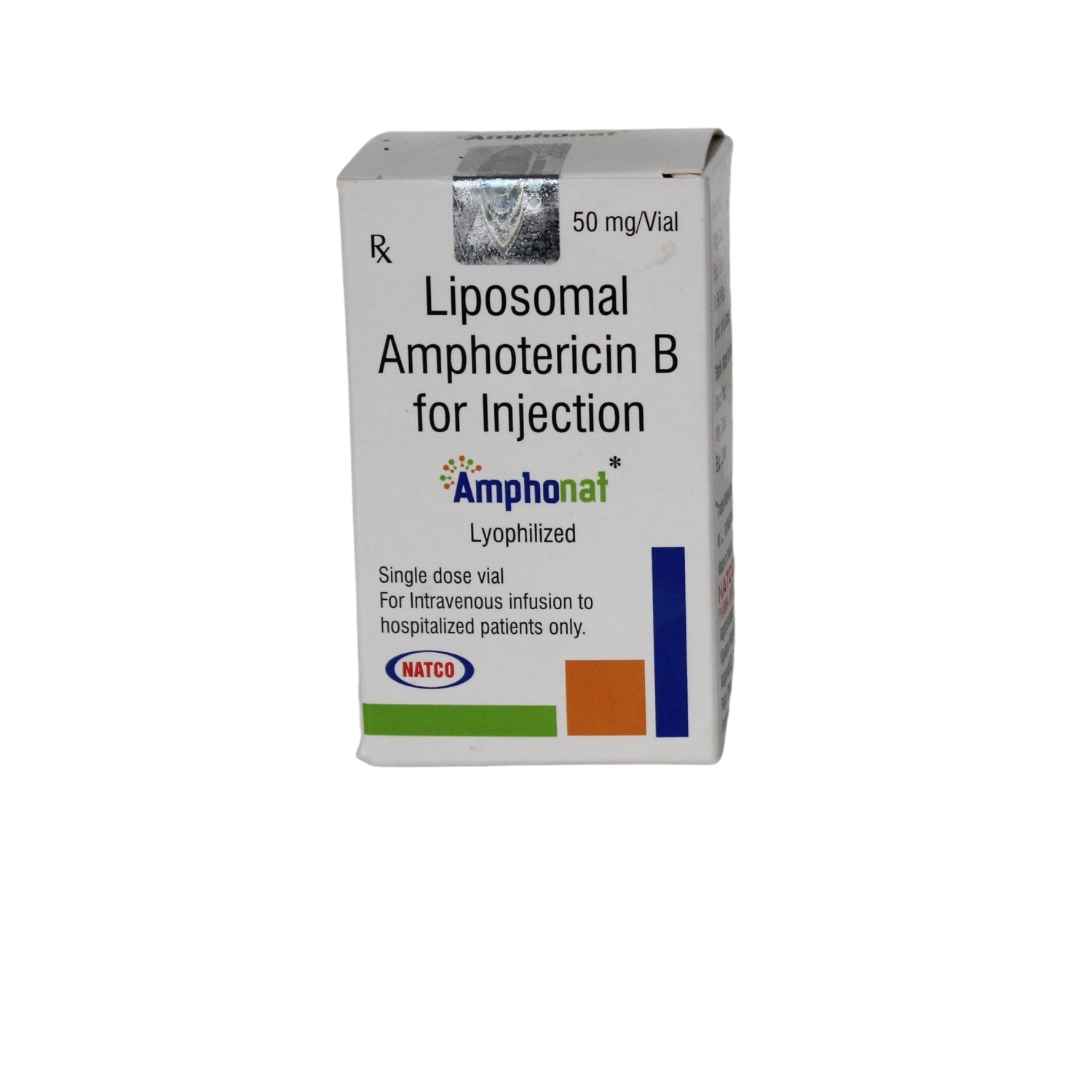
Amphonat 50 mg Injection is a sterile, lyophilized powder containing Liposomal Amphotericin B, designed for intravenous infusion. The liposomal encapsulation of Amphotericin B improves its safety profile by reducing renal toxicity and infusion-related reactions. This formulation allows for higher dosing and prolonged circulation time, enhancing its efficacy against deep-seated fungal infections and visceral leishmaniasis.
By binding to ergosterol in fungal cell membranes, Amphotericin B alters membrane permeability, causing leakage of intracellular components and subsequent fungal cell death. The liposomal delivery system targets the drug to infected tissues while minimizing exposure to healthy cells, thereby reducing adverse effects.
Indications
Amphonat 50 mg Injection is indicated for:
- Severe Systemic Fungal Infections: Including infections caused by Aspergillus, Candida, and Cryptococcus species.
- Visceral Leishmaniasis: A parasitic disease affecting internal organs.
Composition
Each vial contains:
- Active Ingredient: 50 mg of Liposomal Amphotericin B.
- Excipients: Hydrogenated soy phosphatidylcholine, cholesterol, distearoyl phosphatidylglycerol, and other liposomal components.
Dosage and Administration
- Reconstitution:
- Reconstitute the lyophilized powder with 12 mL of Sterile Water for Injection to obtain a concentration of 4 mg/mL.
- Gently swirl the vial until the powder is completely dissolved.
- Dilution:
- Further dilute the reconstituted solution with 5% Dextrose Injection to achieve the desired concentration.
- Do not use saline or other diluents, as they may cause precipitation.
- Administration:
- Administer the diluted solution via intravenous infusion over a period of 30 to 120 minutes, depending on the dose and patient tolerance.
- Monitor the patient for any infusion-related reactions during administration.
Note: Dosage varies based on the type and severity of the infection, as well as patient factors such as weight and renal function. Consult the prescribing information for detailed dosing guidelines.
Precautions
- Hypersensitivity: Contraindicated in patients with known hypersensitivity to Amphotericin B or any component of the formulation.
- Renal Impairment: Use with caution in patients with pre-existing renal dysfunction; monitor renal function regularly during therapy.
- Electrolyte Imbalance: Monitor and correct electrolyte disturbances, particularly hypokalemia and hypomagnesemia, before and during treatment.
- Infusion-Related Reactions: Be vigilant for symptoms such as fever, chills, hypotension, and respiratory distress during infusion. Pre-medication with antipyretics or antihistamines may be considered to reduce these reactions.
- Pregnancy and Lactation: Use during pregnancy only if the potential benefit justifies the potential risk to the fetus. Caution is advised when administered to nursing mothers.
Storage
- Store unopened vials in a refrigerator at 2°C to 8°C (36°F to 46°F).
- Protect from light; keep vials in the original carton until use.
- Do not freeze.
- After reconstitution, the solution should be used promptly. If not used immediately, it can be stored for up to 24 hours at 2°C to 8°C.
- Monitoring: Regularly monitor renal function, liver function, and serum electrolytes during therapy.
- Drug Interactions: Be aware of potential interactions with other nephrotoxic agents or drugs that can cause electrolyte imbalances.
- Patient Education: Inform patients about the importance of reporting any adverse reactions promptly and adhering to scheduled laboratory tests.